
Meet Xstim SPINE FUSION STIMULATOR
Introducing
Xstim SpinE Fusion Stimulator
Our flagship product, Xstim Spinal Fusion Stimulator, is a class 3, FDA-approved device whose electrical stimulation signal has been clinically proven to promote bone healing after lumbar spinal fusion surgery for one or two levels (1,4). This proven capacitive coupling technology safely and effectively triggers the body's inherent healing mechanism through the transmission of low-level electrical pulses to the fusion site (1).
-
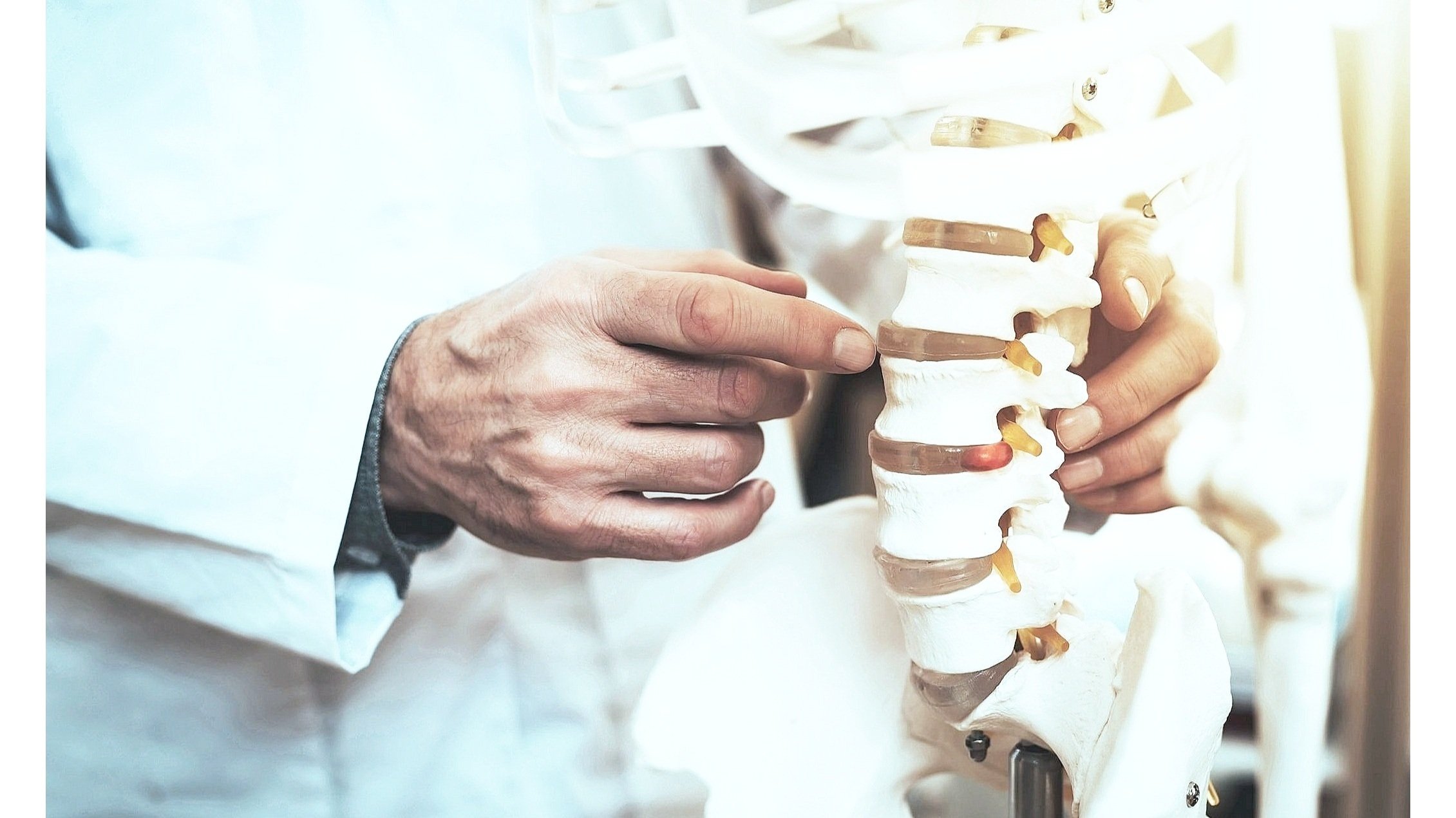
CLINICALLY PROVEN SAFE & EFFECTIVE
Backed by robust clinical evidence and recommended for use by the North American Spine Society to advance spine fusion healing in posterolateral fusions. (3)
-

Compact design with built-in technology
100% modern-day wearable. Our low profile, lightweight, modern design discreetly fits comfortably under clothes or bracing options. Large high-definition onboard screen makes reviewing treatments easy, with no companion apps to download and manage.
-

Concierge care and support
At Xstim, we redefine the way you experience bone growth stimulation medical device support. We understand the trust you put in our team and are committed to the best healthcare experience for both patient and prescriber.
designed to promote
natural healing from within, harnessing the body's own Restorative processes to support improved outcomes for patients.
Reference: (1,2)
Xstim Spine Fusion Stimulator Components
Learn more about the
Xstim SPINE FUSION STIMULATOR
Healing Advantage
Dive deeper into understanding how Xstim spine fusion stimulation therapy can play a crucial role in helping you achieve lumber fusion.
LEARN MORE:
References: (1) Goodwin CB, Brighton CT, Guyer RD, Johnson JR, Light KI, Yuan HA. A double-blind study of capacitively coupled electrical stimulation as an adjunct to lumbar spinal fusions. Spine. 1999;24(13):1349-1356. (2) Wang Z, Clark CC, Brighton CT. Up-regulation of bone morphogenetic proteins in cultured murine bone cells with use of specific electric fields. J Bone Joint Surg Am. 2006;88(5):1053-1065. (3) https://www.spine.org/Product-Details?productid=%7B3D71338D-AF81-E611-851E-005056AF031E%7D (4) U.S. Food and Drug Administration. (2023). PMA P230025 FDA Summary of Safety and Effectiveness Data.









